If you want to draw Co molecular orbital diagram, first find you know atomic number
Atomic Numbers:
Atomic number of Carbon (C): 6
Atomic number of Oxygen (O): 8
Adding the atomic numbers of Carbon and Oxygen: 6 + 8 = 14
Electronic Configurations of carbon and oxygen:
Electronic configuration for Carbon (C): 1s² 2s² 2p²
Electronic configuration for Oxygen (O): 1s² 2s² 2p⁴
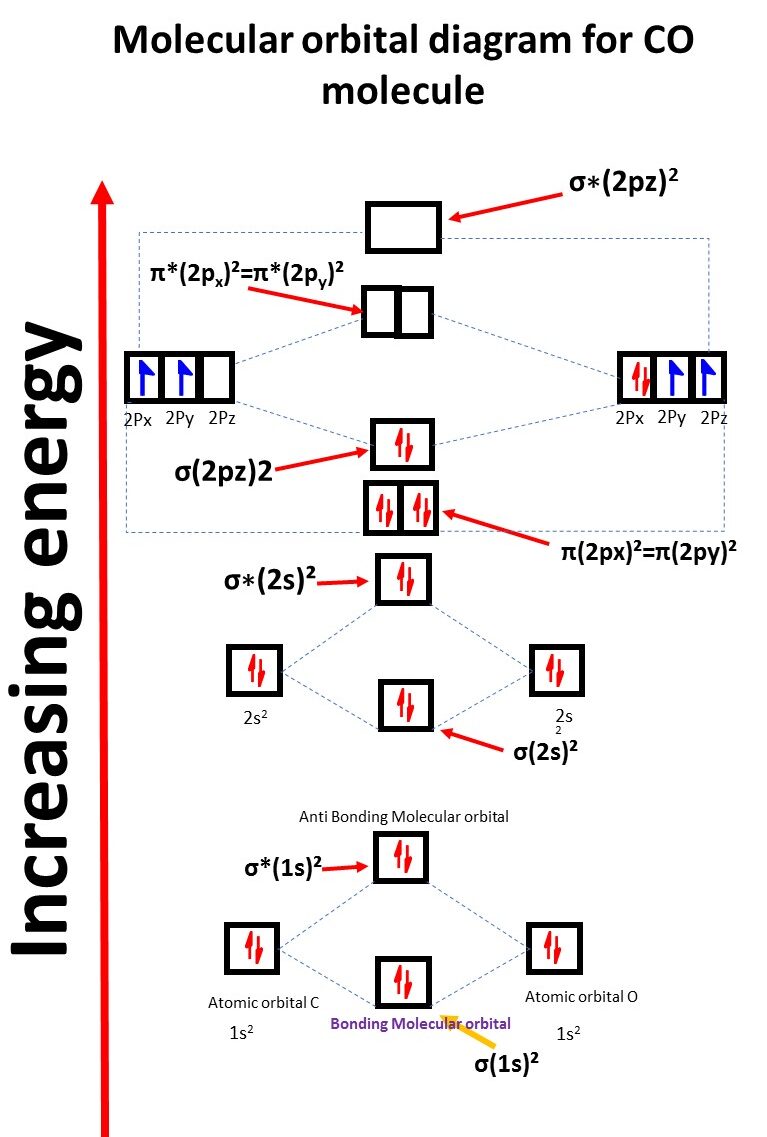
Carbon Monoxide CO Molecular Orbital Diagram
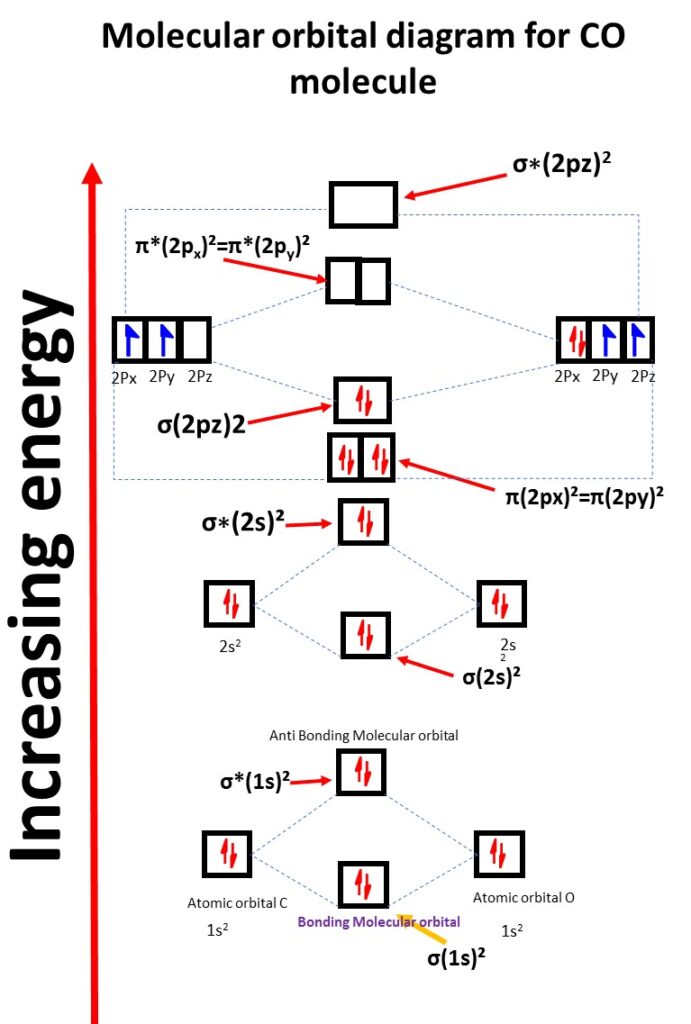
Co molecular orbital electronic configuration
σ(1s)², σ*(1s)², σ(2s)², σ*(2s)², π(2px)2=π(2py)2 , σ(2pz)²,
Magnetic Nature of CO
The carbon dioxide (CO) molecule is diamagnetic in nature. Diamagnetic substances have all their electrons paired, leading to a cancellation of magnetic moments. Since all the electrons in the molecular orbital diagram for CO are paired, the molecule does not exhibit a net magnetic moment and is diamagnetic.
It’s clear that you understand the concepts of atomic and molecular structures. If you have any more questions or need further clarification, feel free to ask! Click here.
See more information about Co molecular orbital diagram, click here