Question CN- molecular orbital diagram
Answer CN- molecular orbital diagram (cyanide ion), which consists of carbon and nitrogen atoms,
follow these steps:
Atomic Numbers:
The atomic number of carbon (C) is 6, and for nitrogen (N) it is 7. Adding these together and accounting for the negative charge (-1) gives us a total of 14 electrons.
Electronic Configurations:
The electron configurations for carbon and nitrogen are as follows:
Carbon (C): 1s² 2s² 2p²
Nitrogen (N): 1s² 2s² 2p³
CN- Molecular Orbital Diagram:
Below is the molecular orbital diagram for CN- with its corresponding electronic configuration:
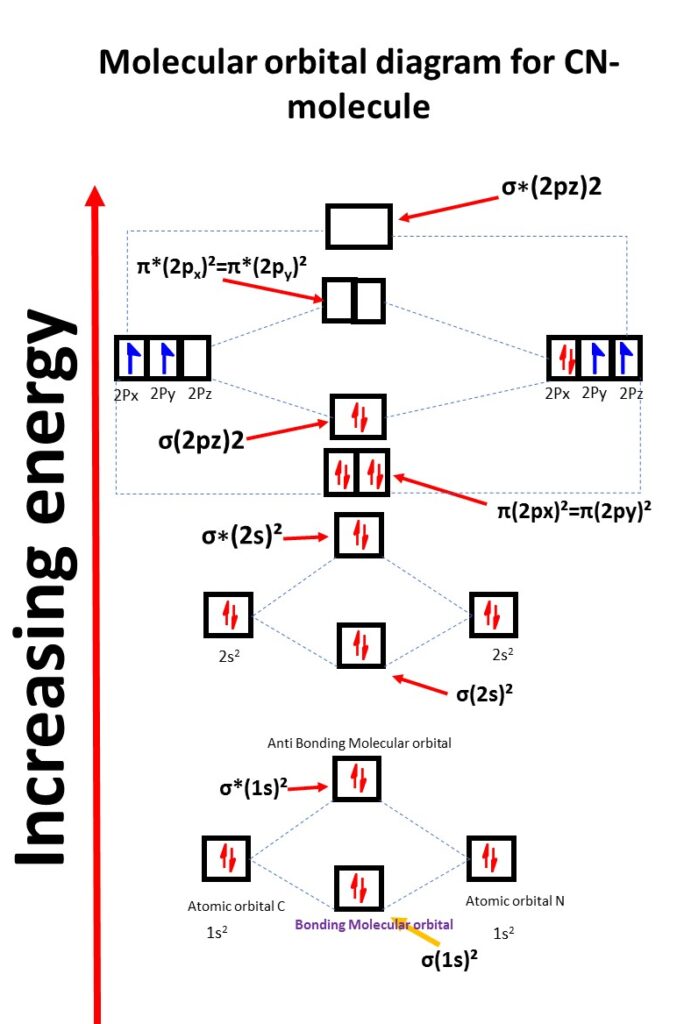
In this diagram, the molecular orbitals are listed in order of increasing energy. The electron configuration of CN- is distributed among these orbitals.
Additionally, it’s worth noting that the CN- molecule is diamagnetic in nature. This is because all of its electrons are paired up in molecular orbitals, resulting in no net magnetic moment.
CN- molecular orbital diagram
the CN- molecule has a total of 16 electrons, and they fill the molecular orbitals accordingly.
σ(1s)², σ*(1s)², σ(2s)², σ*(2s)², π(2px)2=π(2py)2 , σ(2pz)²,
click here for more molecular orbital diagram
click here for more information about CN molecular orbital diagram